The American Cancer Society (ACS) has officially endorsed a new method for cervical cancer screening, offering women an alternative to the traditional Pap smear. This update reflects ongoing advances in early detection technology and aims to make cervical cancer screening more accessible and effective.
The New Screening Option
The newly recommended alternative involves a self-administered HPV test, which detects high-risk strains of the human papillomavirus (HPV) that are most commonly linked to cervical cancer. Unlike the conventional Pap smear, which requires a clinic visit and professional sample collection, the self-swab test allows individuals to collect a sample at home and send it to a laboratory for analysis.
Why the Change Matters
Cervical cancer remains a significant health concern, though rates have dropped dramatically in countries with effective screening programs. Experts note that many women still face barriers to traditional Pap testing, including:
- Lack of access to healthcare providers.
- Discomfort or anxiety about in-clinic procedures.
- Time constraints or transportation challenges.
The ACS highlights that self-administered HPV testing can overcome many of these barriers, potentially increasing participation in cervical cancer screening programs and identifying at-risk individuals earlier.
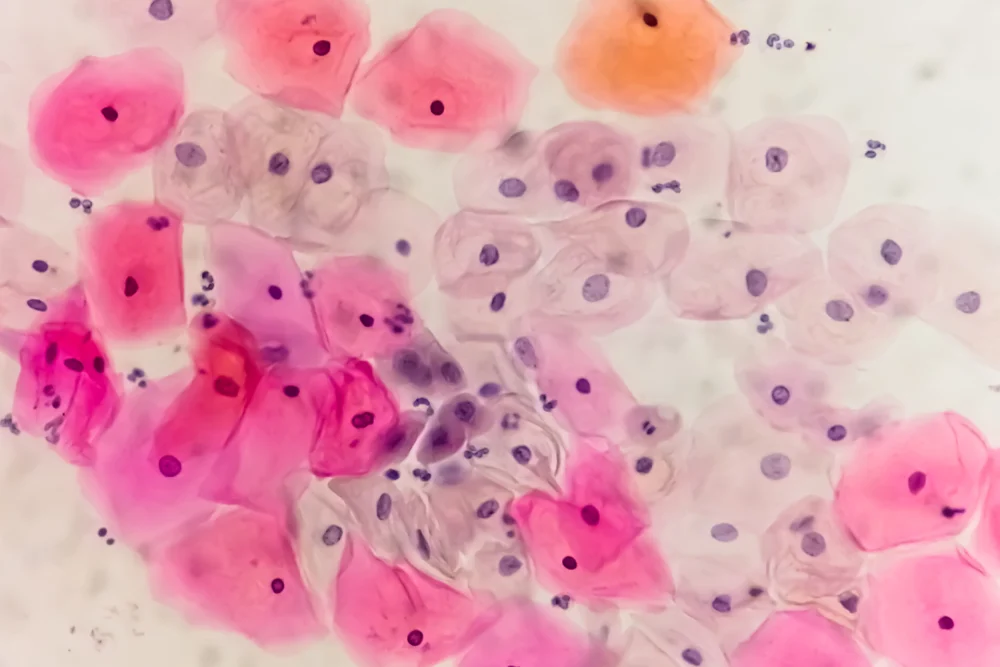
Effectiveness of the Self-Swab Test
Clinical studies show that self-collected HPV samples are nearly as accurate as clinician-collected samples for detecting high-risk HPV strains. Early detection is critical, as identifying HPV infections before they develop into cervical cancer allows for timely intervention, significantly improving outcomes.
Dr. Lisa Martinez, a gynecologic oncologist, emphasized, “Offering a home-based testing option empowers women to take control of their health while maintaining the same standard of care in cancer prevention.”
Implementation and Accessibility
The ACS recommends that women discuss their screening options with healthcare providers to determine which method is most appropriate based on age, medical history, and risk factors. Several programs are expected to roll out mail-in HPV testing kits, making the process convenient and confidential.
Looking Ahead
The endorsement represents a broader trend toward personalized and flexible healthcare solutions, leveraging technology to improve early cancer detection. As adoption grows, experts anticipate higher screening rates and, ultimately, fewer cases of advanced cervical cancer.








Leave a Reply